Introduction
Ethylene oxide (EtO, C₂H₄O, CAS: 75-21-8) is a colorless, flammable gas with a slightly sweet odor and belongs to the family of aliphatic epoxides. This carcinogenic compound is primarily used in the production of ethylene glycol (antifreeze) and various industrial applications, including pesticides and as a sterilization agent for medical equipment and plastic devices that cannot be sterilized by steam.
EtO is carcinogenic to humans through inhalation. Primary sources of EtO emissions include uncontrolled industrial emissions or venting. Recent studies have also identified wastewater systems as a potential source of EtO exposure due to processes, spills, or leaks. Standard wastewater treatment processes often fail to effectively remove EtO. Consequently, industrial facilities need a reliable method to monitor EtO in their wastewater. To address this, the EPA contracted Enthalpy Analytical – Houston to develop a GCMS method for EtO detection in wastewater using EPA method 8260.
Development
Chromatographic Performance
Some of the main challenges in Ethylene oxide (EtO) analysis using EPA method 8260 are its low boiling point and its spectra being nearly identical to those of non-target compounds such as Acetaldehyde.
Several different GC columns with varying stationary phases and dimensions, as well as different instrument parameters were tested until achieving optimal chromatographic performance that exhibited (a) adequate and reproducible peak shape, (b) sufficient peak resolution from other compounds and (c) linearity throughout increasing concentrations.
Additionally during this stage of the method development process, several areas of efficiency improvement were developed to reduce run times and costs associated with analysis.
Qualitative Identification
With our method’s peak resolution of Acetaldehyde and EtO, the retention time of EtO can also be used to help verify correct qualitative identification.
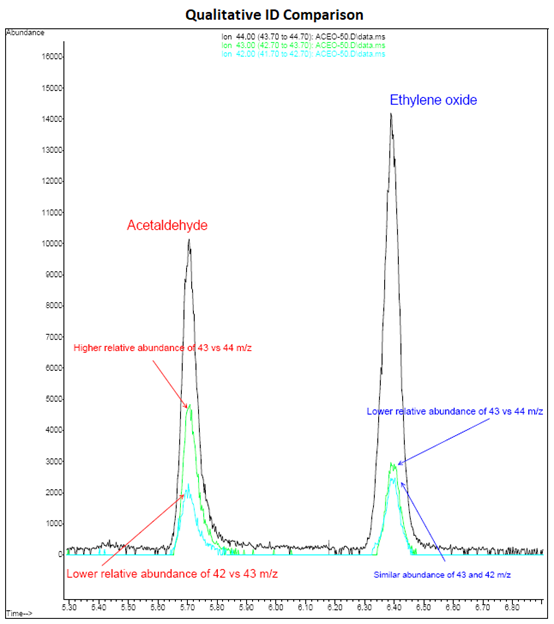
EtO’s mass spectra shares characteristic ions with other non-target compounds which can lead to challenges with correctly identifying and quantitating EtO.
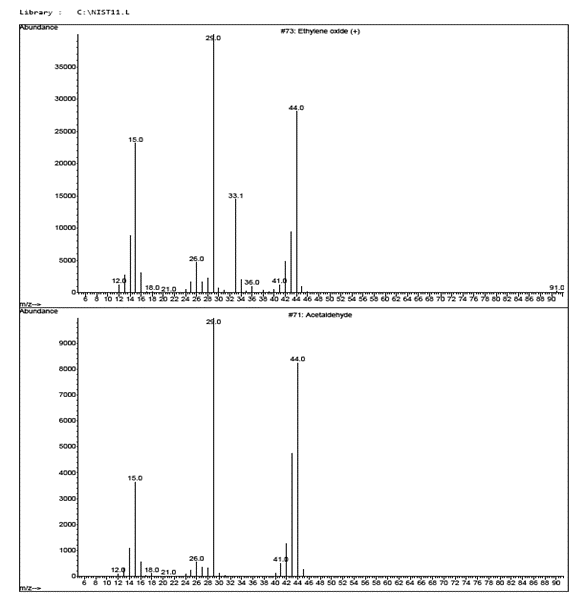
Note from the figure above that EtO can be distinguished from Acetaldehyde by the abundance ratios of 43 m/z relative to the 42 m/z and 44 m/z ions.
Analytical Performance
Calibration and Quantitation
The 44 m/z ion was chosen for quantitation after demonstrating that background abundances of 44 m/z were negligible. For the internal standard, a solution of Ethylene oxide-d4 was used.
Using this “labeled” compound as the internal standard provides quantitation results that in effect “correct” for purging, chromatographic, and matrix behavior of the analog EtO. This also eliminates the need for traditional surrogate evaluation as the labeled internal standard abundances serve as the ideal surrogate.
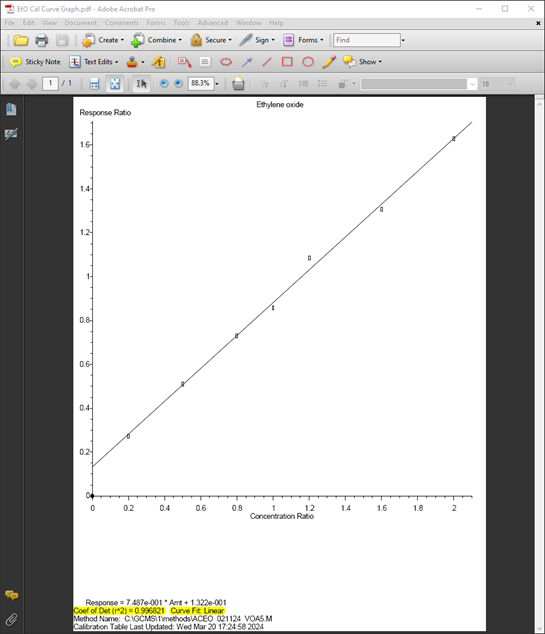
The calibration range for ethylene oxide was analyzed from 20 to 200 ug/L, using an internal standard concentration of 100 ug/L. Although the Response Factor RSDs were slightly above 20%, adequate linearity was achieved using a linear curve fit.
Cal Concentrations, ug/L
| 20 | 50 | 80 | 100 | 120 | 160 | 200 |
|---|
Peak Performance
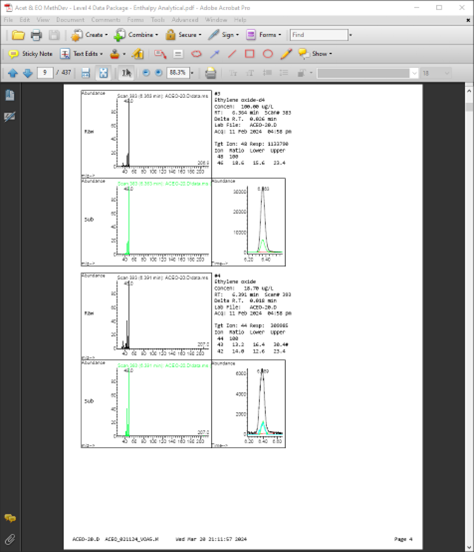
Ethylene oxide – d4 @ 100 ug/L and Ethylene oxide @ 20 ug/L
The following table shows the reproducibility of Ethylene oxide-d4, used as the internal standard at a concentration of 100 ug/L.
Ethylene oxide-d4 Abundances
| ICAL 1 | ICAL 2 | ICAL 3 | ICAL 4 | ICAL 5 | ICAL 6 | ICAL 7 | Std Dev |
|---|---|---|---|---|---|---|---|
| 1133790 | 1100735 | 1104970 | 1073646 | 981603 | 1077477 | 1024396 | 5% |
MDL for Ethylene oxide in Water
| Compound | Spk, ug/L |
Rep 1 | Rep 2 | Rep 3 | Rep 4 | Rep 5 | Rep 6 | Rep 7 | Rep 8 | Avg. | Std Dev. |
MDL, ug/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene oxide | 20 | 17.93 | 18.28 | 17.09 | 17.70 | 16.28 | 18.01 | 18.95 | 20.40 | 18.08 | 1.23 | 3.68 |
Spike Recovery for Ethylene oxide in Water
| Compound | Spk, ug/L |
Rep 1 %R |
Rep 2 %R |
Rep 3 %R |
Rep 4 %R |
Rep 5 %R |
Rep 6 %R |
Rep 7 %R |
Rep 8 &R |
Avg. %R |
Std Dev. %R |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethylene oxide | 20 | 90 | 91 | 85 | 89 | 81 | 90 | 95 | 102 | 90 | 6 |
In Conclusion
The Hazardous Organic NESHAP Maximum Achievable Control Technology (HON MACT) rule requires certain covered facilities, including Synthetic Organic Chemical Manufacturing Industry (SOCMI) facilities, to perform ongoing fenceline monitoring using new and existing EPA methods for air (M327) and wastewater (8260).
The detection and monitoring of ethylene oxide in industrial wastewater are crucial for ensuring environmental safety and public health. Enthalpy Analytical – Houston has developed an advanced GCMS method using EPA method 8260 to address this need, overcoming significant analytical challenges. Currently, the Texas Commission on Environmental Quality (TCEQ) does not offer accreditation of ethylene oxide by EPA method 624.1.
By partnering with Enthalpy Analytical, industrial facilities can effectively monitor and mitigate EtO emissions, adhering to regulatory standards and protecting their communities. To learn more about our cutting-edge methods and how we can assist with your environmental analysis needs, visit Enthalpy Analytical today. Let us help you ensure a safer and cleaner future.


