How Absorption and Adsorption Affects VOC Behavior
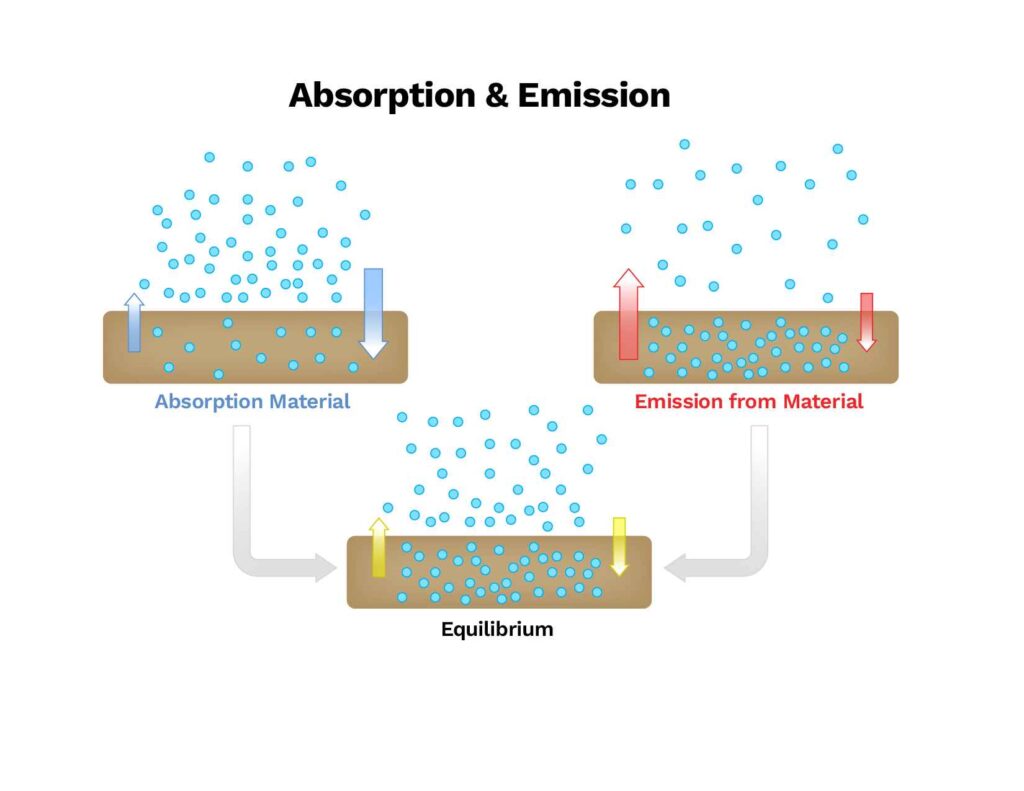
Volatile Organic Compounds (VOCs) gases are moved throughout the air space, onto surfaces, into porous materials, and back out again through Absorption (inside of), Adsorption (surface only) and Emission. Absorption of VOCs by materials is driven by concentrations of these gases in the air. When the concentration in the air is greater than that of the materials, the VOCs will migrate into the materials via their surfaces. Gases are then emitted back into the air from the materials’ surfaces, a process controlled by both the surface and porosity of the materials interior. Gas migrates from within the material to the surface, where some is emitted into the surrounding air.
This cycle continues as long as there is an imbalance between the concentrations of VOCs in the materials and surrounding air, resulting in a long-term, low level of emissions from the surface into the air. Eventually, the amount within the material will reach such a low level that the amount at the surface and the amount in the air are similar, eliminating the imbalance. Even after this point, the surface and air continue to exchange gases equally in a process referred to as dynamic equilibrium.
How Fire and Smoke Affects VOC Behavior
During and after a fire, large numbers of reaction products including soot/char/ash particulates, as well as hundreds of chemical compounds, are released into the air. While some of these compounds dissipate quickly and are not a long-term contamination concern, others can linger for weeks, months, or even years, causing concern about chronic effects and uncertainty regarding the efficacy of the cleanup or remediation process.
Both fire and the chemical compounds produced move through several stages. Initial chemical reaction products are dominated by high concentrations of small, reactive compounds like carbon monoxide and dioxide, formaldehyde, acrolein, cyanide, which typically dissipate quickly due to their high reactivity and volatility.
Larger and less reactive compounds, though less concentrated, persist over a longer period of time and are the cause of many post-fire complaints. These comprise almost every class of chemical – oxygenated compounds like aldehydes, esters, acids, and alcohols, aromatics like benzene, and heavier compounds like polycyclic aromatic hydrocarbons (PAHs), as well as a range of hydrocarbons created by lower temperature processes or remnants of the original fuel material (incomplete burn).
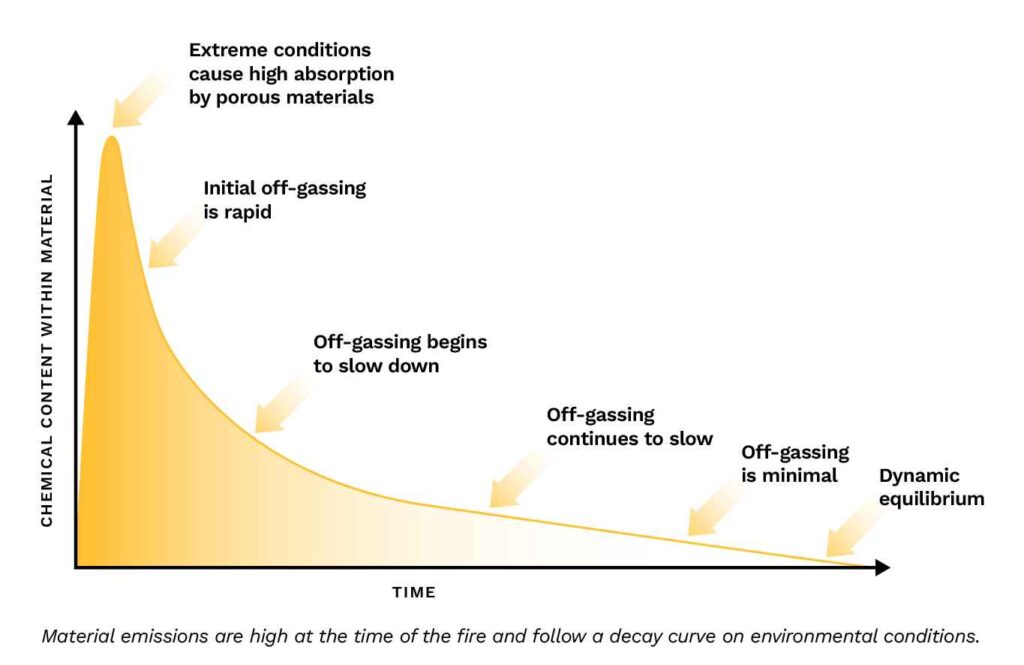
The heat of a fire drastically increases the number of compounds in the air of the surrounding building, creating an imbalance between the air and nearby surfaces and materials, and pushing compounds deeper into these materials.
Why Do the Odors Return?
The concern with VOCs is their penetration into porous materials. Imagine how long the smell of smoke or fire residue lingers in clothing and fabrics, even after a simple backyard barbeque. This is caused by the adsorption of VOCs into the fabrics during or shortly after the fire and their subsequent emission back into the indoor air.
The process of adsorption and emission, or off-gassing, is disproportionate in that the adsorption occurs over a very brief period, while off-gassing can take months or even years to reach an undetectable level. So, why the disparity? Fundamentally, it comes down to equilibrium and the relative concentration proportions. During a fire, the concentrations of VOCs in the air are extremely high, while those in the building’s materials are low or nonexistent, causing the materials to quickly “soak up” the fire emissions. After the fire is out and the smoke has dissipated, the concentrations in the air are more moderate, and, after a few days, very low. At this point, the opposite condition has occurred: the concentrations in the materials are now much higher than in the air. But rather than quickly resolving the imbalance—as had occurred during the extreme conditions of the fire—the process is much slower due to the moderate “normal” conditions.
Environmental conditions have a significant effect on how long post-fire contamination remains. As ventilation increases, the chemical concentration in the air is essentially diluted. Temperature and humidity have the opposite effect, where higher temperature and humidity will shift the equilibrium to emission. So, on warm, humid days, the smoky odor may be more noticeable than in colder weather.
How Can You Combat These Odors?
Particulate removal alone is often not sufficient to address smoke odors. Whether in the restoration, insurance, or consulting fields, or for an impacted homeowner, there are many ways to better understand the potential future impact of lingering chemicals from fire and smoke damage.
Enthalpy Analytical offers many different test options for post-structure fire and wildfire events, including assessing for odor complaints, health/exposure concerns, and waste characterization. Please connect with one of our laboratory experts for help developing your next post-fire sampling event.


